Pressure Measurement
Leak Test using Pressure Transducers
Introduction:
Leak tests are often performed on manufactured items before shipment to verify pressure integrity. The most common way of doing this is to pressurize the article, seal it off and wait for some decline in pressure to appear on a pressure gauge. This is often sufficient when testing for gross leaks, but not as fast or effective as may be needed to detect slow leaks. This application note will describe the basics of leak testing and how to configure a differential test system that speeds up the leak test and improves accuracy.
An Example:
A simple example will show how to determine the basic parameters of pressure decay when leak testing with air.
Let’s say an article that is 10 cubic inches in volume must be leak tested at 15 psig to verify that any leak present is less than 0.005 cubic inches per minute. Boyle’s Law is used to determine the relationship of air volume and pressure:
P1 V1 = P2 V2
Where:
P1 = Starting Pressure
P2 = Final Pressure
V1 = Starting Volume
V2 = Ending Volume
Notice that any units of pressure and volume will work, if used consistently.
The Simple Pressure Decay Test
A simple pressure decay test applies the test pressure to the article and then observes any decrease in that pressure over time. Here is how the time required for this test can be determined:
The first step is to calculate how much air will be in the article when it is pressured to the test pressure of 15 psig. We use absolute pressures for this, and rearrange Boyle’s equation as follows:
V1 = (P2 V2) / P1
Where:
V1 = Amount of gas before pressure is applied
V2 = 10 Cu In, final volume of gas
` P1 = 14.7 psia, starting absolute pressure
P2 = 14.7 + 15 = 29.7 psia, final absolute pressure
Solving for V1, the volume required to bring the test article pressure up to 15 psig:
V1 = (29.7 10) / 14.7 = 20.20 cubic inches
So if the pressurized article contains 20.20 Cu In of air at the start of the test, how much must leak out in order to successfully detect the leak? For the simple pressure decay test, we must first know the accuracy of the pressure gauge used to record the change in pressure. A good electronic pressure sensor will have an accuracy of 0.25% over its full scale. If the pressure sensor has a full scale of 15 psig, that error represents a pressure of 0.0375 psi. But if a drop in pressure of 0.0375 psi is reported by the pressure sensor, it may just be error. So a pressure drop several times greater than the error should be specified for the pass/fail criteria. Five times the error would be a pressure of about 0.2 psi, and this would more reliably indicate the presence of a leak
If the maximum leak rate specification is 0.005 Cu Inches/minute, how long would it take for the pressure gauge to register the 0.2 psi pressure drop? To determine this, we first need to know how much air must leak out of the article under test to create a pressure change of 0.2 psi. We use Boyle’s Law again, as follows:
V2 = (P1 V1) / P2
Where:
V2 = Volume needed to hold the new, lower pressure, Cu In
P1 = Initial pressure, 29.7 psia
V1 = Initial volume = 10.0 Cu In
P2 = Pressure after leakage = 29.5 psia
Solving for V2:
V2 = (29.7 10.0) / 29.5 = 10.07 Cu In
The new volume needed to hold the new pressure (without leakage) is 10.07 Cu In. Since the volume of the container has not changed, this means that the escaped air due to leakage is equivalent to 0.07 Cu In. At a maximum allowable leak rate of 0.005 Cu In per minute, it will take something like 14 minutes to verify such a leak using the simple pressure decay test.
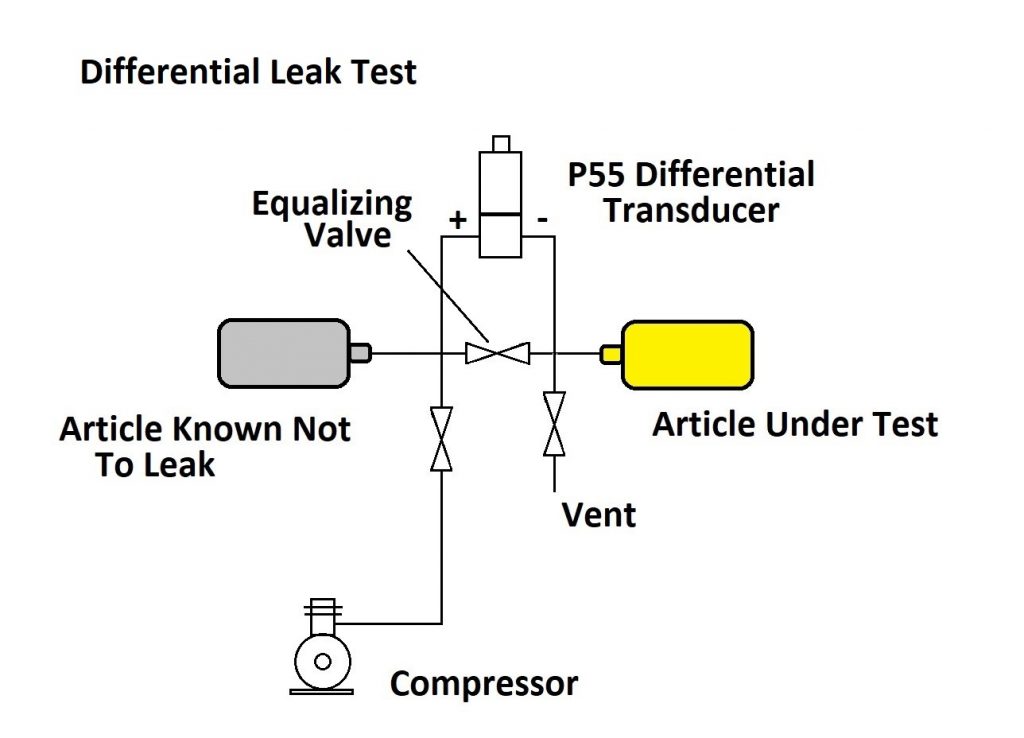
The Differential Pressure Method:
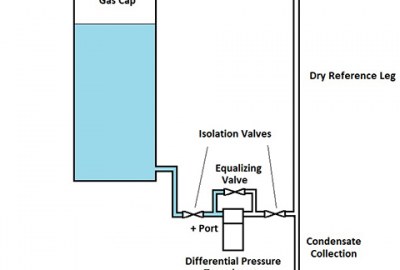
A better method for detecting small leak rates is the differential pressure method. In this scheme, two articles are used: one that is known not to leak and the article that is under test. Both articles are pressured up to the same test pressure and then the two articles are sealed off. A differential pressure sensor is used to detect any change between the pressure in the article known not to leak and the article under test. This can be easily automated. A much more sensitive differential pressure transducer is used for this and small leak rates can be determined much faster.
As shown in the diagram, the test sequence runs as follows:
1 – The equalizing valve is opened and the vent valve closed.
2 – The pressure valve from the compressor is opened, bringing both articles up to the test pressure.
3 – The pressure and equalizing valves are closed, sealing the system, and the differential pressure sensor records any small change in pressure between the two articles.
4 – After enough time has elapsed to measure the leakage, the equalizing valve is opened and the vent valve opened to exhaust the system.
As with the simple pressure decay test, determining the amount of time needed to detect the leak is a matter of knowing how small a pressure drop can be accurately observed by the differential pressure transducer. A good low-pressure transducer like the Validyne P55 pressure transducer or the DP15 pressure sensor is available in full scale pressures of 0.5 psi, with an accuracy of 0.25%.
As before, we determine the pressure drop that can accurately be observed by the differential pressure sensor. A full scale of 0.5 psi and an accuracy of 0.25% means that the error of the measurement is just 0.00125 psi. Allowing for a pressure drop of five times this error to be reliable, the pressure inside the article under test must drop just 0.00625 psi to detect a leak.
Rounding this up to 0.007 psi, we next determine how much air must leak out of the article under test to lower the pressure by 0.007 psi. As before, we use Boyle’s Law:
V2 = (P1 V1) / P2
Where:
V2 = the volume needed to hold the new, lower pressure, Cu In
P1 = the initial pressure, 29.7 psia
V1 = the initial volume = 10.0 Cu In
P2 = the pressure after leakage = 29.693 psia
Solving for V2:
V2 = (29.7 10.0) / 29.693 = 10.002 Cu In
So something like 0.002 Cu In of air exiting out of the article under test is enough to lower the pressure just enough for the leakage to be reliably detected. This will take under one minute to detect the allowable leak rate of 0.005 Cu In per minute. The time saved over the simple pressure decay test is significant.
Some Precautions:
The article known not to leak should be identical to the article under test. When air is compressed it heats up slightly, and this will increase the pressure in both articles. If the heat is dissipated equally by the two articles, the temperature rise will have less of a perturbing effect on the test. With the differential pressure method, extra time should be allotted to allow for some cooling, and this can be done without greatly extending the time of the test. The absolute pressures will increase roughly to the ratio of the increase in absolute temperatures, so if some idea of the temperature rise can be known, adjustments can be made to the time allowed to complete the test. It is also helpful to devise an article that has a leak rate that is known to be exactly equal to the maximum leak specification. This can be used to verify the testing system from time to time.






Leave a reply
You must be logged in to post a comment.